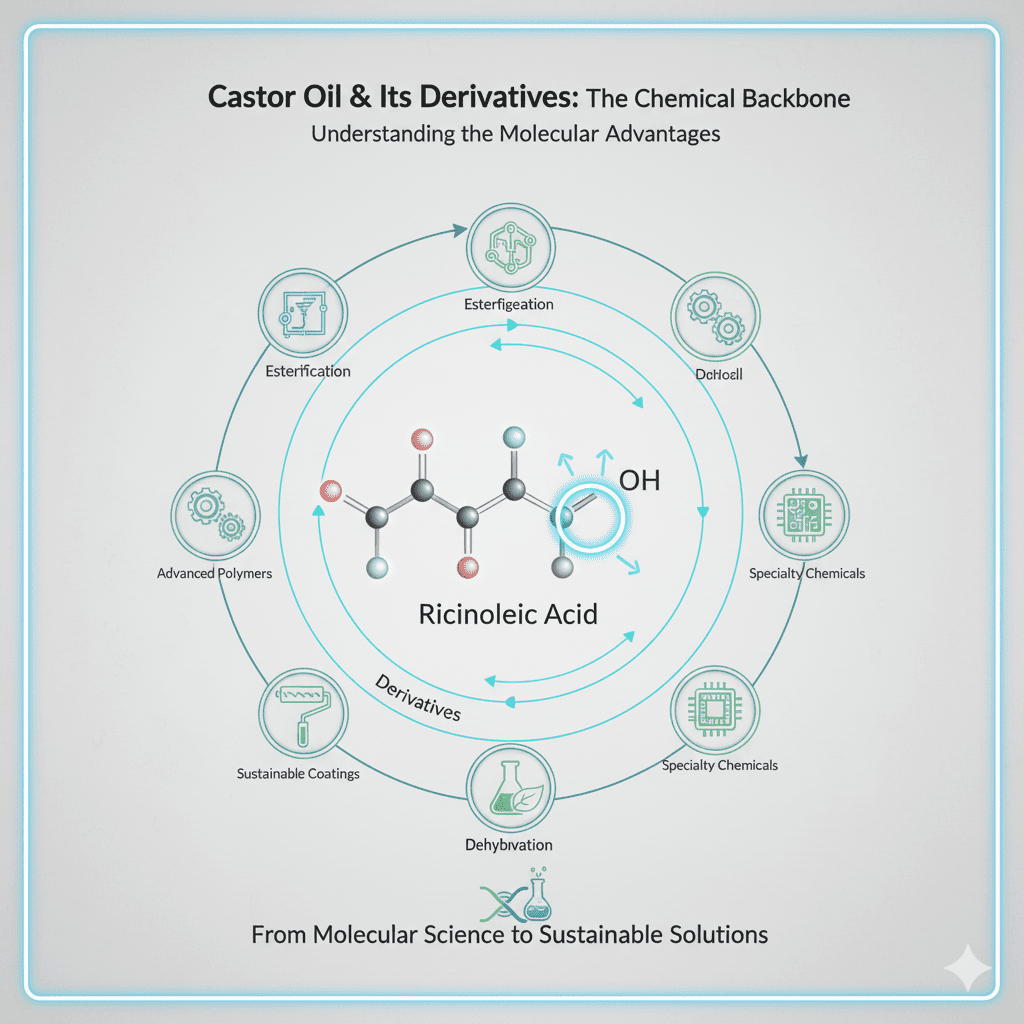
This article would take a deep dive into the molecular science behind castor oil’s versatility. It would explain the significance of the hydroxyl group on ricinoleic acid and how this functional group acts as a crucial site for various chemical reactions (e.g., esterification, ethoxylation, sulfation, dehydration, hydrogenation) that produce an extensive range of derivatives. The article would highlight how these precise molecular modifications enable the creation of tailor-made materials with specific properties, allowing castor oil to serve as a building block for sustainable alternatives in complex chemical industries.
Key Points:
- Detailed explanation of ricinoleic acid’s molecular structure and its hydroxyl group.
- Discussion of key chemical reactions: mechanisms and resulting derivative types.
- How molecular engineering allows for property tuning (e.g., viscosity, solubility, reactivity).
- Examples of how specific derivatives’ molecular structures lead to their industrial applications (e.g., polyols for urethanes, sebacic acid for Nylon-11).
- The scientific basis for castor oil’s superior performance in green chemistry.
- Bridging the gap between natural feedstock and advanced synthetic materials.